DrugCard
DrugCard is an advanced AI tool specifically designed to facilitate local literature screening within the pharmaceutical industry. This innovative tool ensures that drug safety routines are streamlined and fully compliant …
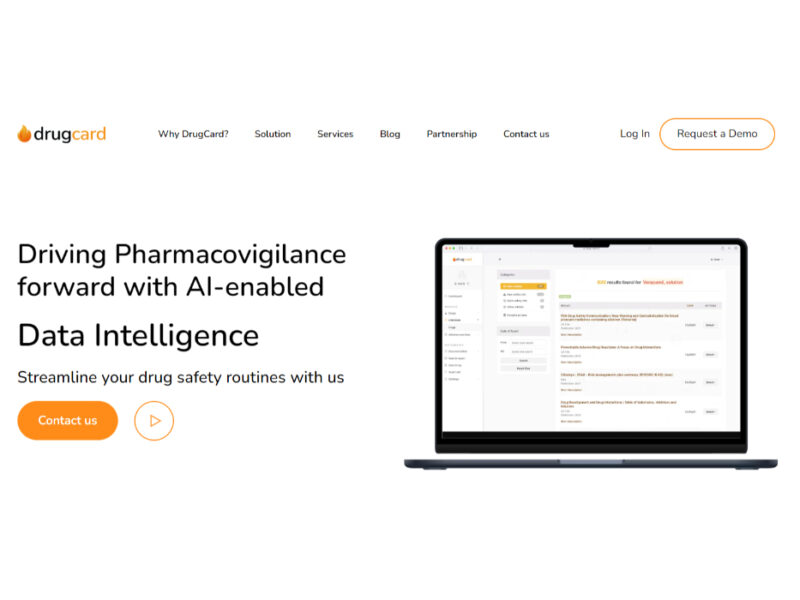
About DrugCard
Use Cases
Use Case 1: Automated Local Literature Screening
Problem: Pharmacovigilance teams manually review hundreds of local medical journals, which is time-consuming and prone to human error.
Solution: DrugCard automates the screening of over 1,817 local journals across 100+ languages, identifying relevant safety information instantly.
Example: A pharmaceutical company uses DrugCard to monitor regional medical publications in 104 countries to identify any mentions of adverse reactions associated with their products.
Use Case 2: Enhancing CRO Project Management
Problem: Contract Research Organizations (CROs) often struggle to maintain high-quality screening standards while managing multiple client projects.
Solution: The platform provides a centralized, scalable solution that allows CROs to manage more pharmacovigilance projects with higher accuracy and 60% time savings.
Example: A CRO implements DrugCard to automate the cover-to-cover reading of journals for five different clients, freeing up staff for high-priority signal detection tasks.
Use Case 3: Regulatory Audit Readiness
Problem: Marketing Authorization Holders (MAHs) face increasing pressure from regulators to provide transparent and traceable documentation of their drug safety routines.
Solution: DrugCard offers a high degree of transparency and traceability, ensuring that all screening activities are logged and compliant with GVP regulations.
Example: During a regulatory audit, an MAH quickly generates comprehensive reports from DrugCard to prove their literature monitoring process is holistic and compliant.
Key Features
- Automated local literature screening
- Multi-language support for 100+ languages
- Monitoring of 1,800+ medical journals
- Traceable and transparent audit trails
- Integrated adverse event database
- Real-time regulatory intelligence tracking
- eCTDlight electronic documentation management
- Multi-region supplier and market scalability